NR 503 Week 8 Final Exam Study Guide; Chapter 7-8
-
$25.00
| Institution | Chamberlain |
| Contributor | Sherrie |
Chapters 7-8
- Question: Which of the following statements best describe efficacy?
- Question: A study is conducted for a pharmaceutical agent that has shown promise for reducing heart disease among women. In order to more fully test the agent, an additional study is done restricting the participants to be randomized to those who have a history of hypertension. Which of the following advantages cannot be claimed by the researchers?
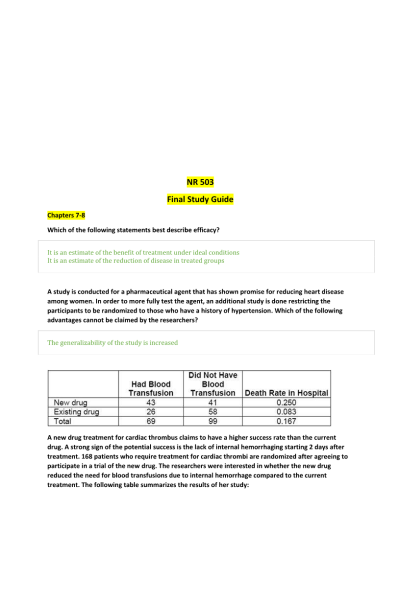
- Question: A new drug treatment for cardiac thrombus claims to have a higher success rate than the current drug. A strong sign of the potential success is the lack of internal hemorrhaging starting 2 days after treatment. 168 patients who require treatment for cardiac thrombi are randomized after agreeing to participate in a trial of the new drug. The researchers were interested in whether the new drug reduced the need for blood transfusions due to internal hemorrhage compared to the current treatment. The following table summarizes the results of her study:
What is the incidence of needing a blood transfusion in the group of persons who were randomized to the new drug treatment? - Question: A new drug treatment for cardiac thrombus claims to have a higher success rate than the current drug. A strong sign of the potential success is the lack of internal hemorrhaging starting 2 days after treatment. 168 patients who require treatment for cardiac thrombi are randomized after agreeing to participate in a trial of the new drug. The researchers were interested in whether the new drug reduced the need for blood transfusions due to internal hemorrhage compared to the current treatment. The following table summarizes the results of her study:
What is the number of persons who died in hospital in the study? - Question: A new drug treatment for cardiac thrombus claims to have a higher success rate than the current drug. A strong sign of the potential success is the lack of internal hemorrhaging starting 2 days after treatment. 168 patients who require treatment for cardiac thrombi are randomized after agreeing to participate in a trial of the new drug. The researchers were interested in whether the new drug reduced the need for blood transfusions due to internal hemorrhage compared to the current treatment. The following table summarizes the results of her study:
What is the main advantage of the randomization of the 168 study participants to one of the two drug treatment groups? - Question: A new drug treatment for cardiac thrombus claims to have a higher success rate than the current drug. A strong sign of the potential success is the lack of internal hemorrhaging starting 2 days after treatment. 168 patients who require treatment for cardiac thrombi are randomized after agreeing to participate in a trial of the new drug. The researchers were interested in whether the new drug reduced the need for blood transfusions due to internal hemorrhage compared to the current treatment. The following table summarizes the results of her study:
The researchers interpret the findings to conclude that the new drug treatment is more likely to result in a blood transfusion and subsequent death. This statement is: - Question: A randomized, double-blind clinical trial of a varicella vaccine observed an estimated incidence of 25% chickenpox episodes in persons receiving the vaccine, compared to 80% among persons receiving a placebo. The estimated efficacy of the vaccine is:
- Question: A multicenter double-blind randomized study was carried out to compare the effect of drug X with that of a placebo in patients surviving acute myocardial infarction (AMI). Treatment with the drug started 7 days after infarction in 1,884 patients, 52% of all persons who were evaluated for entry into the study. 945 participants were randomized to treatment with drug X while 939 were assigned to the placebo group. Patients were then followed for 12 months for reinfarction. There were 152 deaths in the placebo group and 98 in the group receiving drug X.
After entry into the study, patients were first classified into three groups, those who had a previous AMI, those with a first AMI who were at high risk for other cardiovascular diseases such as congestive heart failure, and those with a first AMI who were at low risk for other cardiovascular diseases. Which term best describes the study design? - Question: A multicenter double-blind randomized study was carried out to compare the effect of drug X with that of a placebo in patients surviving acute myocardial infarction (AMI). Treatment with the drug started 7 days after infarction in 1,884 patients, 52% of all persons who were evaluated for entry into the study. 945 participants were randomized to treatment with drug X while 939 were assigned to the placebo group. Patients were then followed for 12 months for reinfarction. There were 152 deaths in the placebo group and 98 in the group receiving drug X.
After assignment to treatment group, 77% of those in the placebo group were men, while 80% of those in the drug X group were men. Which statement is most likely to be true? - Question: A multicenter double-blind randomized study was carried out to compare the effect of drug X with that of a placebo in patients surviving acute myocardial infarction (AMI). Treatment with the drug started 7 days after infarction in 1,884 patients, 52% of all persons who were evaluated for entry into the study. 945 participants were randomized to treatment with drug X while 939 were assigned to the placebo group. Patients were then followed for 12 months for reinfarction. There were 152 deaths in the placebo group and 98 in the group receiving drug X.
A preliminary analysis was conducted after 6 months and found that 87% of participants in the placebo group and 85% of those in the drug X group had taken more than 90% of their prescribed dosages. Which statement best describes this finding? - Question: A multicenter double-blind randomized study was carried out to compare the effect of drug X with that of a placebo in patients surviving acute myocardial infarction (AMI). Treatment with the drug started 7 days after infarction in 1,884 patients, 52% of all persons who were evaluated for entry into the study. 945 participants were randomized to treatment with drug X while 939 were assigned to the placebo group. Patients were then followed for 12 months for reinfarction. There were 152 deaths in the placebo group and 98 in the group receiving drug X.
Which of the following statements best describes the reason for conducting the study as a double-blind trial? - Question: A multicenter double-blind randomized study was carried out to compare the effect of drug X with that of a placebo in patients surviving acute myocardial infarction (AMI). Treatment with the drug started 7 days after infarction in 1,884 patients, 52% of all persons who were evaluated for entry into the study. 945 participants were randomized to treatment with drug X while 939 were assigned to the placebo group. Patients were then followed for 12 months for reinfarction. There were 152 deaths in the placebo group and 98 in the group receiving drug X.
The researchers conclude that treatment with drug X reduces mortality in patients who have had an AMI. The researchers are: - Question: The following data come from a study of approaches to smoking cessation. Smokers who want to quit were randomized to one of four groups: control group C who received no intervention assistance, quitting guide group Q who received brochures about how to quit smoking, quitting guide and support group QS who received quitting brochures as well as social support brochures listing benefits of smoking cessation, and telephone support group T who received the brochures and a monthly phone call from a counselor. Participants received mailed surveys at 8, 16, and 24 months after randomization. The results after 2 years are in the table below. What is the overall quit rate after 2 years of follow-up?
- Question: The following data come from a study of approaches to smoking cessation. Smokers who want to quit were randomized to one of four groups: control group C who received no intervention assistance, quitting guide group Q who received brochures about how to quit smoking, quitting guide and support group QS who received quitting brochures as well as social support brochures listing benefits of smoking cessation, and telephone support group T who received the brochures and a monthly phone call from a counselor. Participants received mailed surveys at 8, 16, and 24 months after randomization. The results after 2 years are in the table below. Which group had the least success in terms of quitting smoking?
- Question: The following data come from a study of approaches to smoking cessation. Smokers who want to quit were randomized to one of four groups: control group C who received no intervention assistance, quitting guide group Q who received brochures about how to quit smoking, quitting guide and support group QS who received quitting brochures as well as social support brochures listing benefits of smoking cessation, and telephone support group T who received the brochures and a monthly phone call from a counselor. Participants received mailed surveys at 8, 16, and 24 months after randomization. The results after 2 years are in the table below. What is the main purpose of randomization in this study?
| Instituition / Term | |
| Term | Spring Session |
| Institution | Chamberlain |
| Contributor | Sherrie |