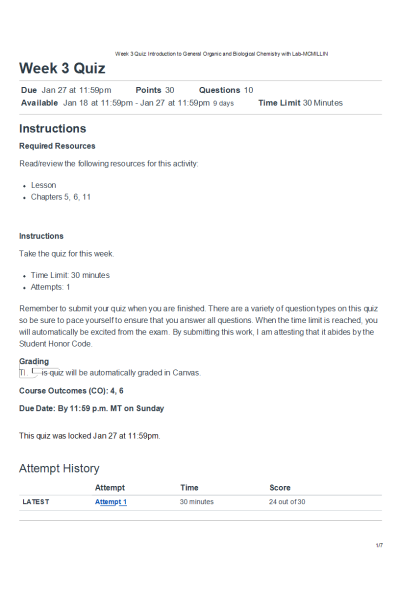
CHEM 120 Week 3 Quiz
-
$20.00
| Institution | CHEM 120 Introduction to General, Organic, and Biological Chemistry with Lab |
| Contributor | Karin Austin |
- Question: Zinc metal will react with aqueous hydrochloric acid to produce aqueous zinc chloride and hydrogen gas. Which of the following is the complete, balanced equation for this reaction?
- Question: Determine the molar mass of the molecule Boron Tri fluoride.
- Question: In the figure shown, is a chemical reaction occurring?
- Question: Balance the following skeletal equation: Li(s) + H2O(l) → LiOH(aq) + H2(g).
- Question: How many atoms of O are in one molecule of the material Al(NO3)3
For the reaction:
- Question: 3Cu(NO3)2(aq) + 2Na3PO4(aq) → Cu3(PO4)2(s) + 6NaNO3(aq)
Select all the following true statements. There may be multiple correct choices.
- Question: Aluminum reacts with oxygen according to the following reaction: 4Al(s) + 3O2(g) → 2Al2O3(s) If 12 moles of aluminum react
completely with oxygen, how many moles of Al2O3 should form?
- Question: What is the % concentration of a solution consisting of 60 grams of solute dissolved in solution with a total volume of 500 ml?
- Question: A solution is prepared by dissolving 8.5 grams of HBr in water to a total volume of 750 mL. What is the molarity (M) of this solution? The
molar mass of HBr is 80.91 g/mol. Show all work.
- Question: You want to prepare a 1.2 M FeCl3 solution. You only have 6 mL of a
3.6 M FeCl3 on hand. How many mL of 1.2M FeCl3 solution can you prepare? Show all work.
| Instituition / Term | |
| Term | Spring |
| Institution | CHEM 120 Introduction to General, Organic, and Biological Chemistry with Lab |
| Contributor | Karin Austin |





-80x80.JPG)




-80x80.JPG)